FDA new strategy to improve detection of unsafe imported foods
Enhanced inspections at U.S. ports of entry and foreign food facilities, as well as the use of its mandatory recall authority, are part of a new strategy designed by the FDA to improve its oversight of imported foods.
“… the U.S. imports about 15 percent of its overall food supply from more than 200 countries or territories representing about 125,000 international food facilities and farms,” according to an announcement this afternoon from FDA Commissioner Scott Gottlieb and Frank Yiannas, the agency’s deputy commissioner for food policy and response.
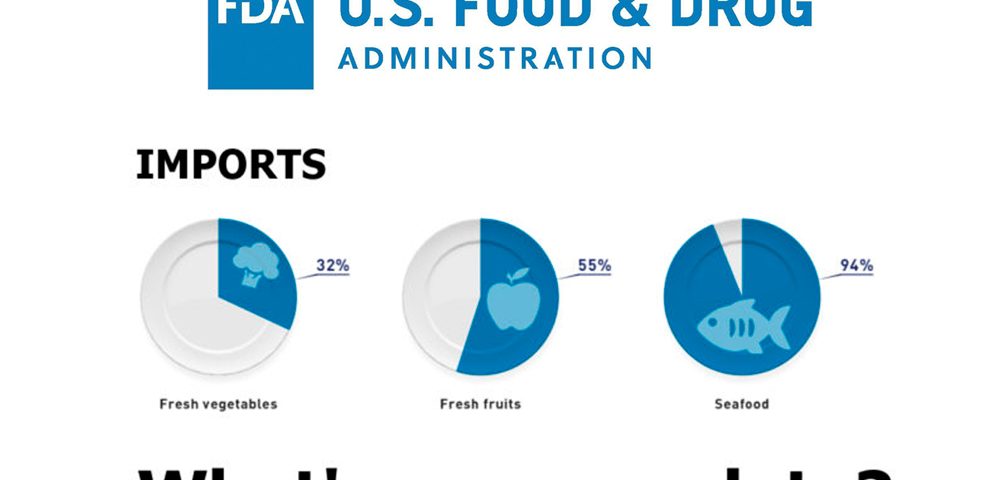
“Over the past 15 years alone, we’ve seen a trend of rising imported foods. Other countries now supply about 32 percent of the fresh vegetables, 55 percent of the fresh fruit and 94 percent of the seafood that Americans enjoy.”
The Food Safety Modernization Act signed into law Jan. 4, 2011, includes requirements for foreign producers and distributors, as well as U.S. importers. The FDA had to develop new rules and go through the mandatory review and revision process before those regulations could go into effect.
In today’s announcement about the Food and Drug Administration’s new strategy, Gottlieb and Yiannas said the more complex worldwide food supply chain has increased the chances of ingredients from a single foreign food manufacturer being used in multiple finished products. That exponentially increases the dangers of foodborne pathogens being introduced to foods available in the United States. The agency’s new strategy will be applied to human and animal foods.
“Our new strategy is designed to meet four important goals: preventing food safety problems in the foreign supply chain prior to entry into the U.S.; effectively detecting and refusing entry of unsafe foods at U.S. borders; responding quickly when the FDA learns of unsafe imported foods; and measuring our progress to ensure that our imported food safety program remains effective and efficient,” Gottlieb and Yiannas said.
“To achieve our first goal of preventing imported safety problems prior to entry into the U.S., we’ll take new steps to continue to ensure that food offered for import meets the same standards as domestically produced food. One of our tools to achieve this goal is onsite inspections of foreign food facilities.”
Technological advancements are providing the FDA with more data than ever to help with its oversight of imports. That data is a crucial factor in the agency’s inspections for the Foreign Supplier Verification Programs (FSVP) rule. The program requires importers to verify that their suppliers are meeting U.S. food safety standards.
In exporting countries where food safety systems and oversight activities are comparable to those in the United States, the FDA should be able to decrease inspections.
“By relying on the activities of these reputable foreign regulatory programs, the FDA can avoid conducting separate inspectional oversight activities. . .” according to today’s announcement. “By leveraging partnerships between the U.S. and other countries with very strong food safety systems through our systems recognition program, we’re able to prioritize our inspection and border screening activities on foods imported from higher-risk areas. In turn, we’re better positioned to verify the safety of food products presented for import.”
The countries already recognized as having comparable food safety systems are Canada, New Zealand and Australia. The FDA is working with the European Union to reach a “mutual assessment.”
Details of the other three goals outlined by the FDA leaders are:
Goal 2 — Detecting and refusing unsafe products by updating our import screening and review processes at the U.S. borders. The FDA intends to incorporate new sources of data from foreign supplier verification programs, voluntary importer incentive programs, accredited third-party auditors, foreign regulatory authorities and domestic supply chain activities.
Goal 3 — To swiftly respond to unsafe imported food, the FDA plans to develop targeted surveillance sampling and testing for the highest-risk products. One way the agency will achieve this goal is the use of its mandatory recall authority.
Goal 4 — The agency will develop an improved global inventory of food facilities and farms to help determine the best allocation of its resources for imported food safety oversight. Identifying areas of higher risk should help the FDA more effectively employ its regulatory tools.
“Overall, our modern strategy is designed to leverage our different authorities and tools to provide a multi-layered, data-driven, smarter approach to imported food safety,” Gottlieb and Yiannas said.
Source: www.foodsafetynews.com


